Banff, Alberta, Canada
SpinaFX, a Canadian company focused on developing minimally invasive, image-guided treatments for back pain caused by contained herniated discs, proudly participated in the 33rd Interdisciplinary Research Conference on Injectable Osteoarticular Biomaterials and Bone Augmentation (GRIBOI 2025). Held from April 10 to 12 at the Fairmont Banff Springs Hotel in Banff, Alberta, GRIBOI brought together global leaders in orthopedics, biomaterials, interventional radiology, and spine care to share research and foster innovation.

Workshop Highlights
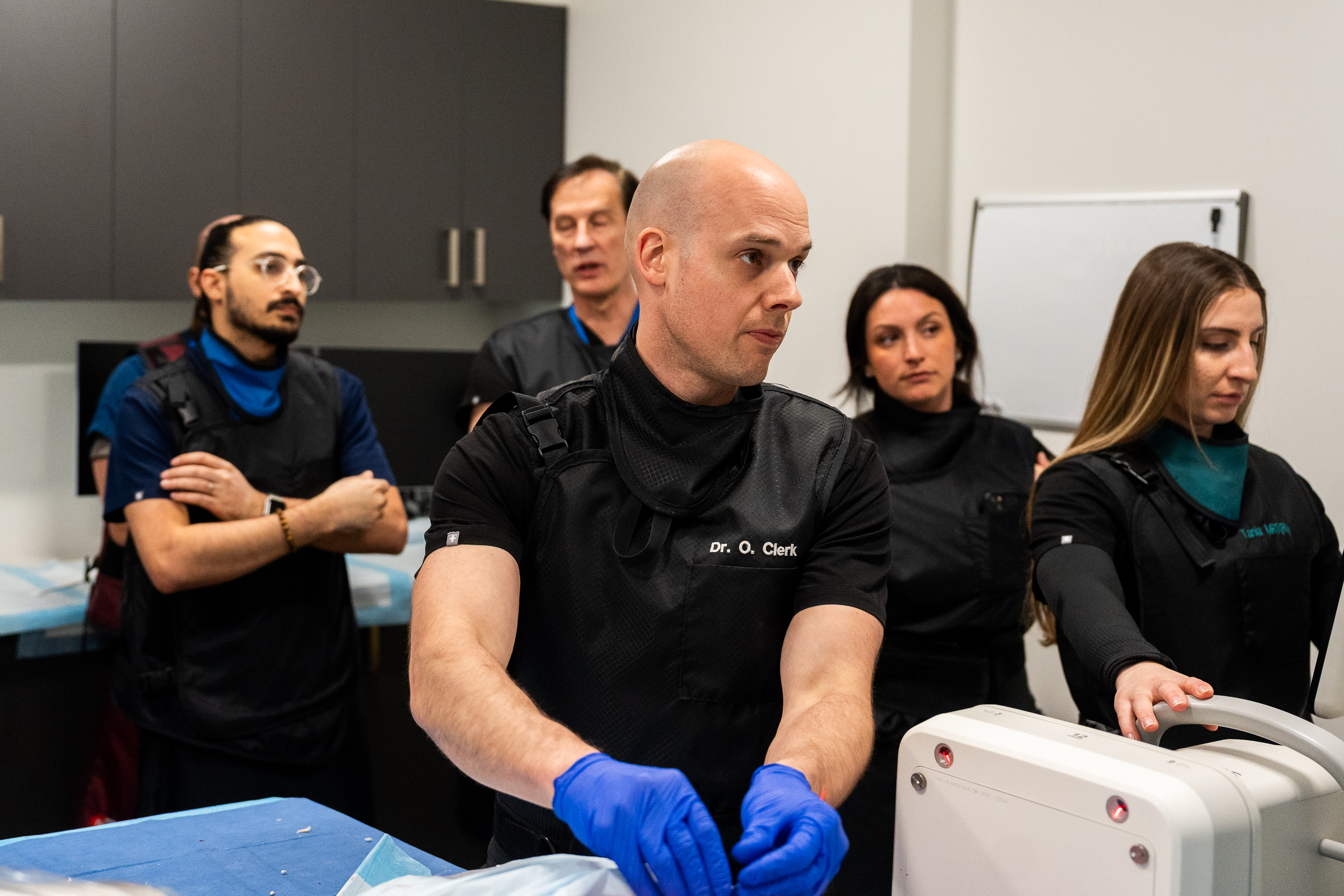
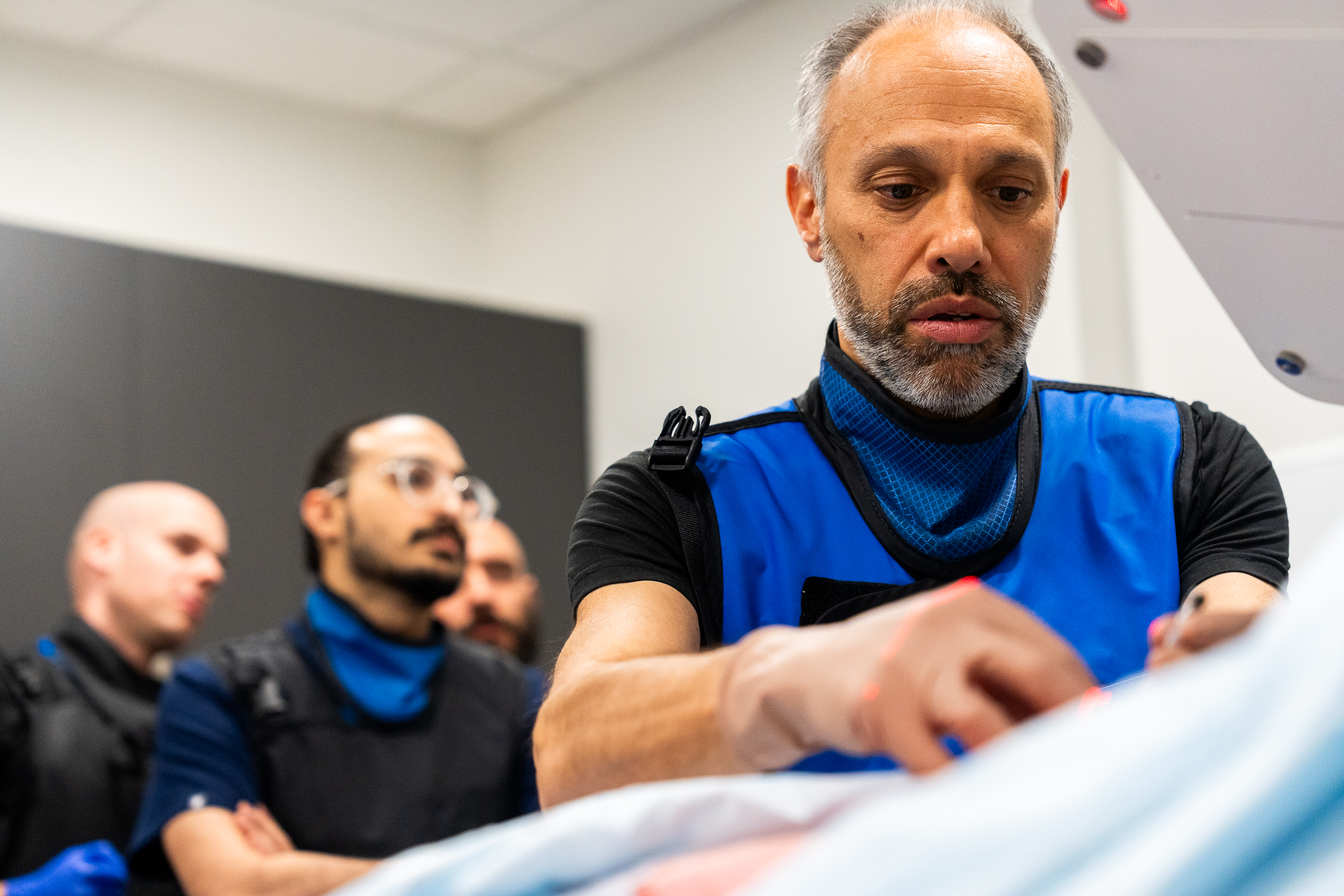
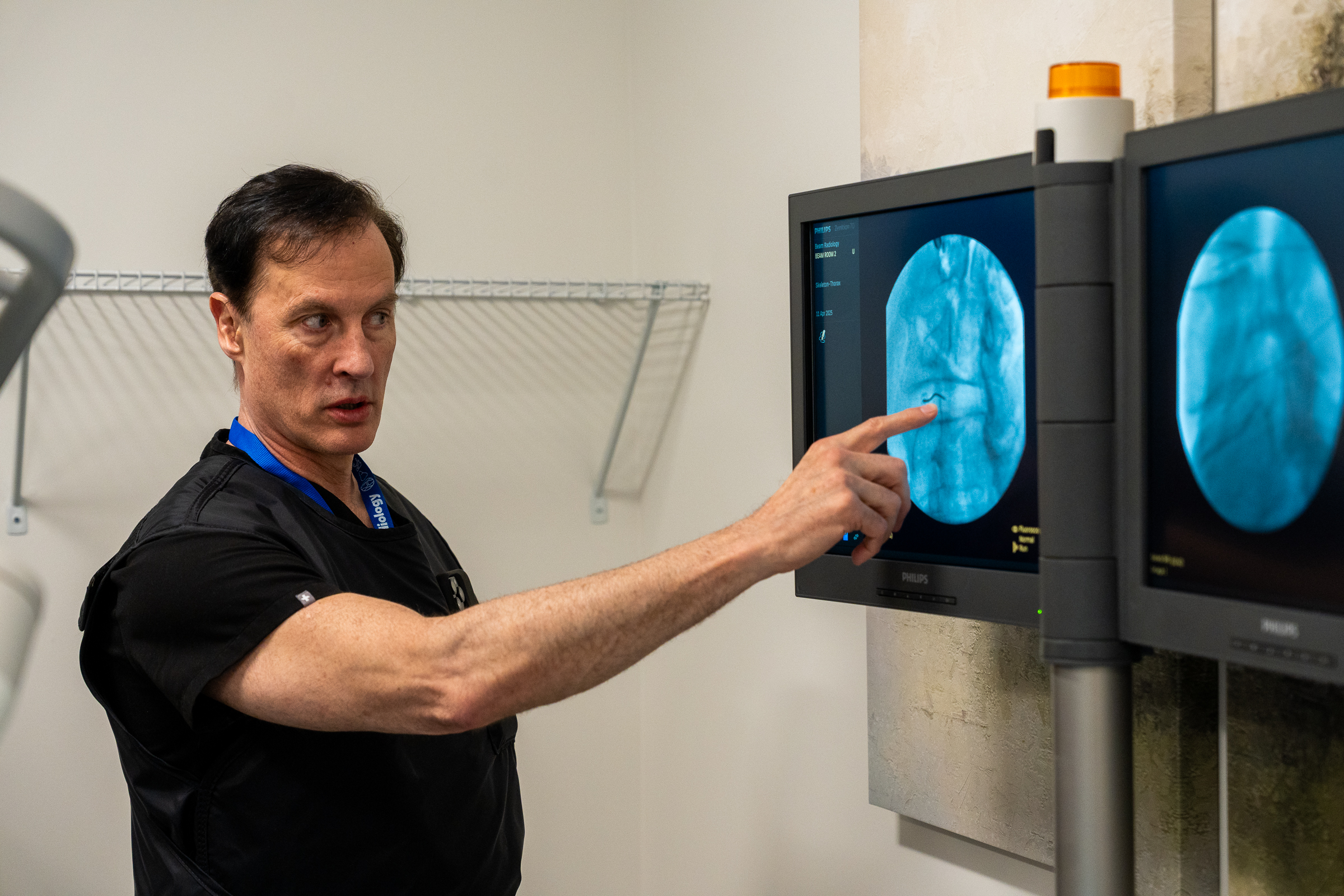
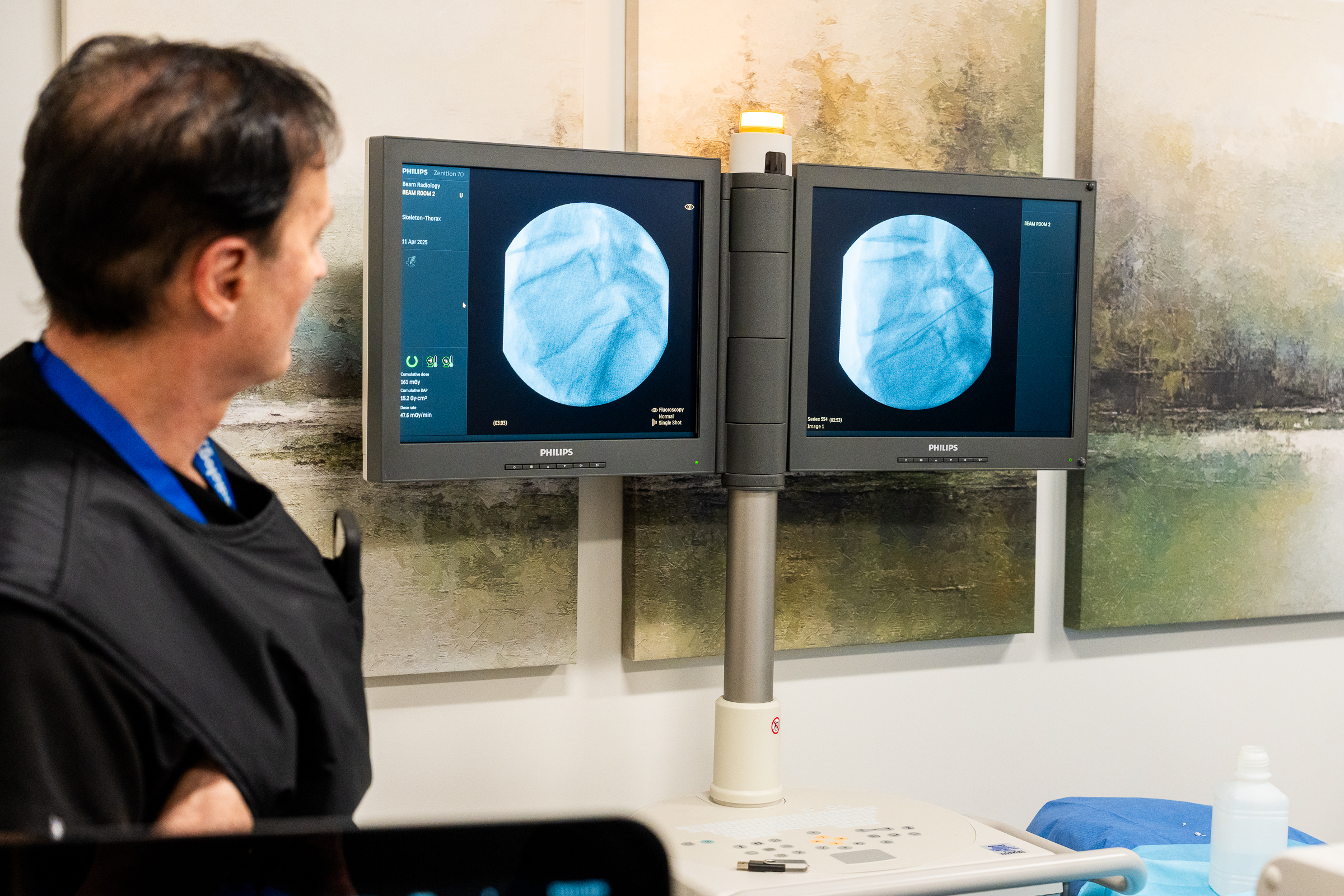
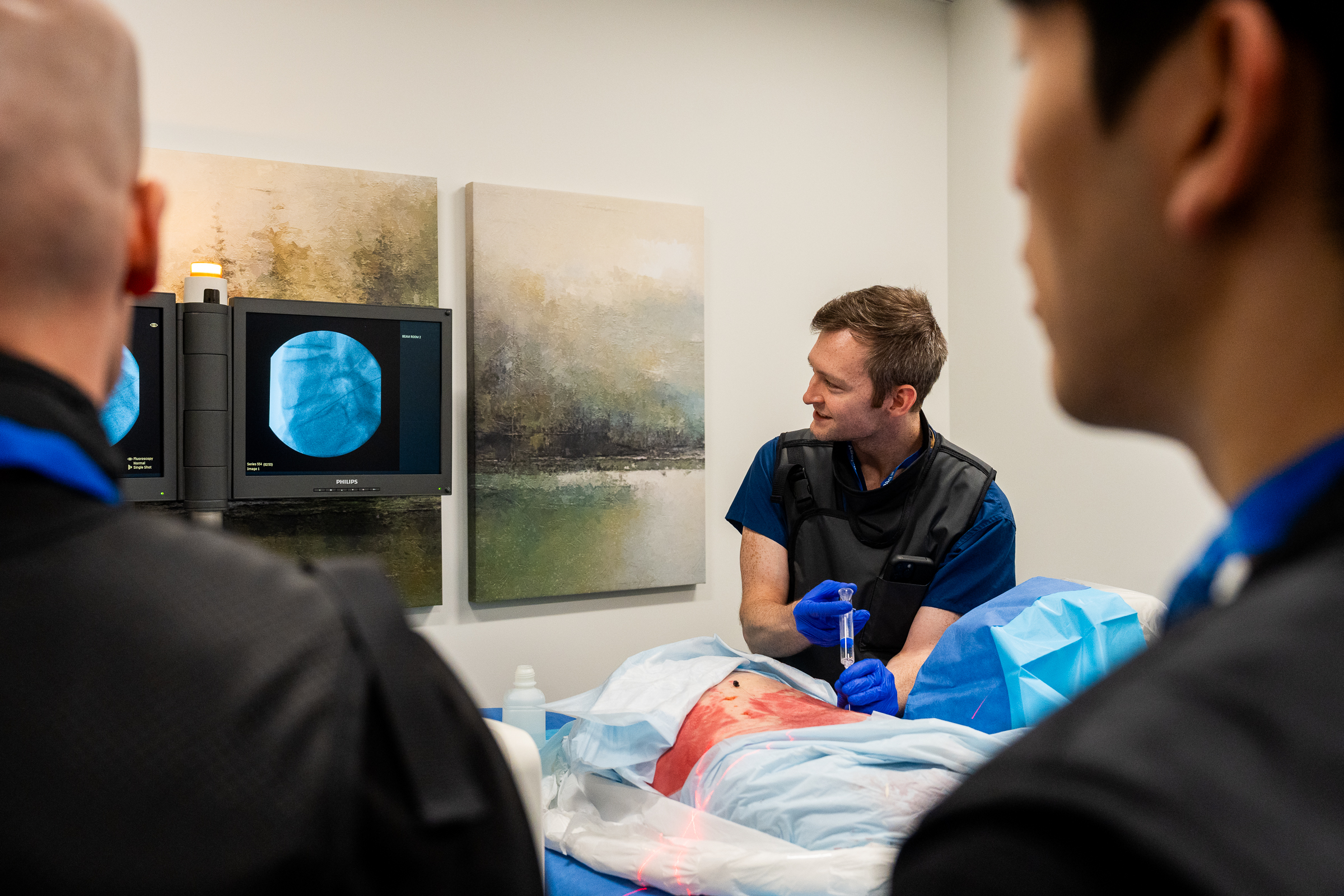
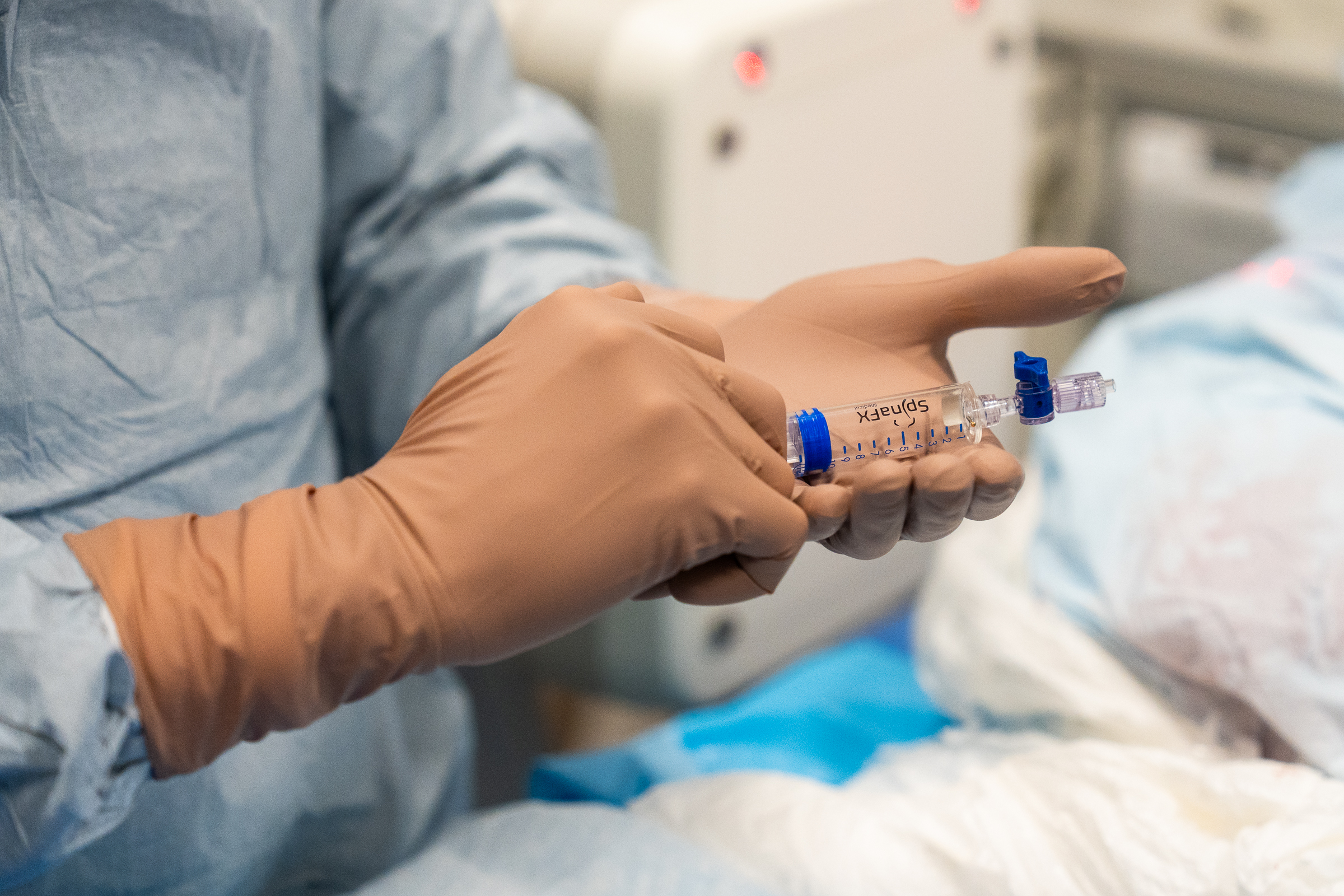
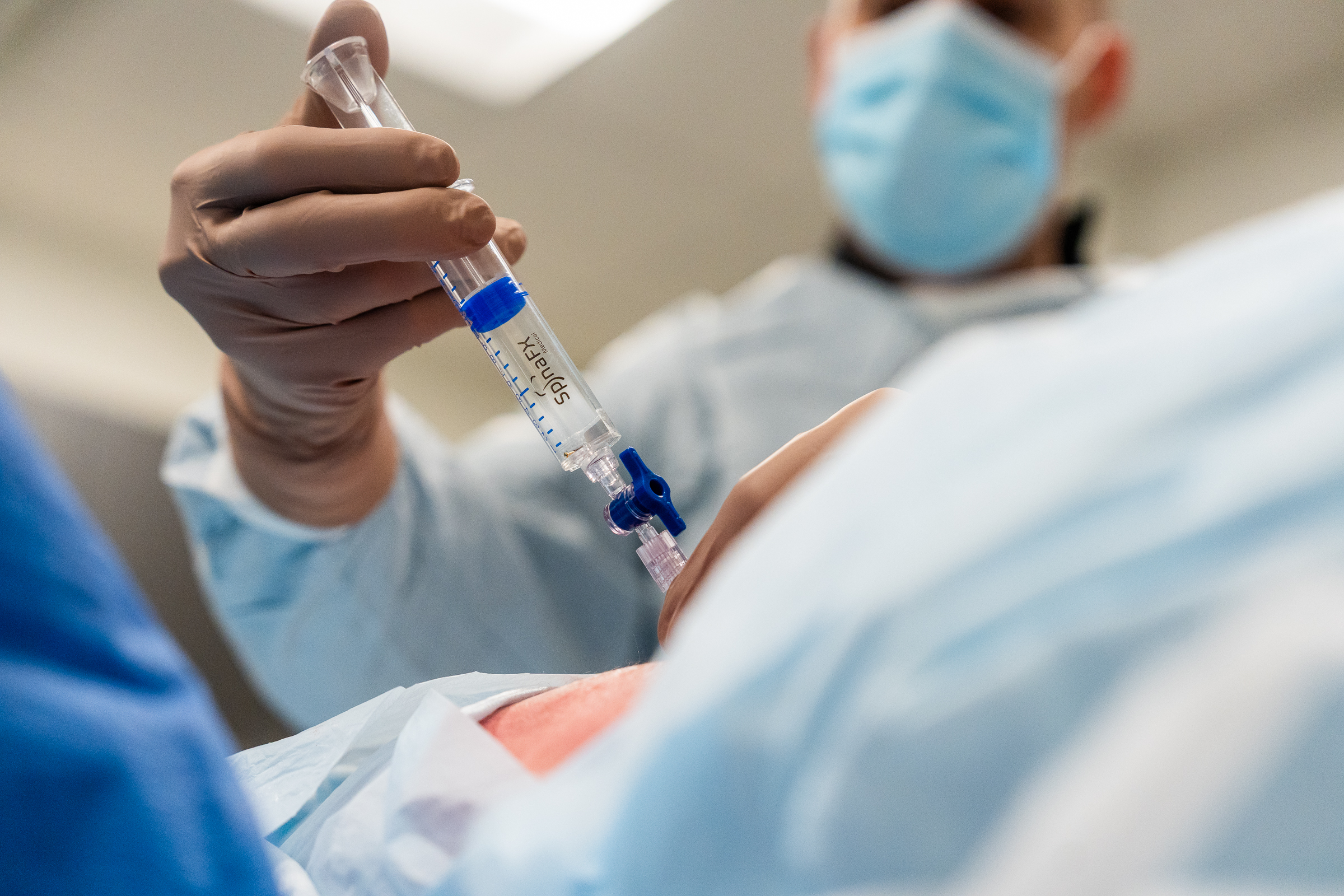
As part of this prestigious event, SpinaFX conducted an educational cadaver lab workshop in Calgary. The session focused on training physicians in the context of Canada’s Special Access Program (SAP), offering an opportunity to explore the technical approach and clinical rationale behind minimally invasive, image-guided procedures for contained lumbar disc herniations.
Participants engaged in hands-on practice with cadaver specimens, gaining valuable insights into the anatomical considerations and procedural techniques involved. The session supported peer-to-peer learning, open discussion, and a deeper understanding of how these procedures might be applied under SAP in Canada.
Key Participants
The workshop was led by Dr. Douglas Beall, Dr. Alexis Kelekis, and Dr. Olivier Clerk — SpinaFX’s distinguished medical advisors and clinical collaborators. Dr. Beall and Dr. Kelekis brought extensive clinical experience and insights to the session, fostering dialogue around the role of advanced imaging and intervention in spinal care.
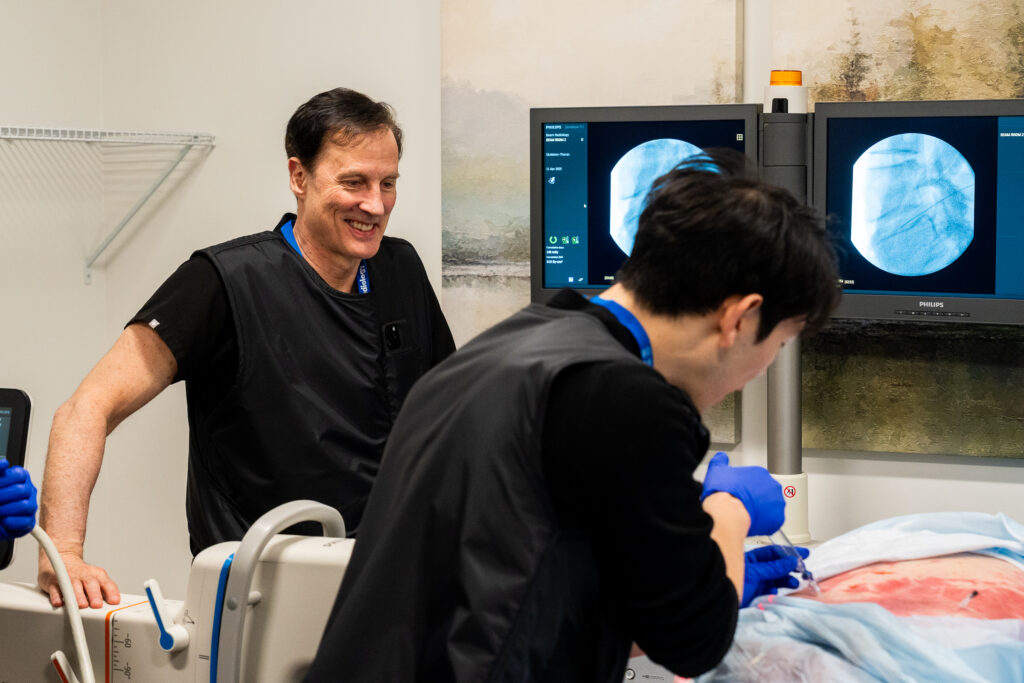
Dr. Olivier Clerk facilitated the session at his Calgary facility. A recognized authority in interventional pain management, Dr. Clerk also shared his real-world experiences using the therapy under SAP conditions, offering valuable perspective on patient selection, procedural nuance, and outcomes observed to date.
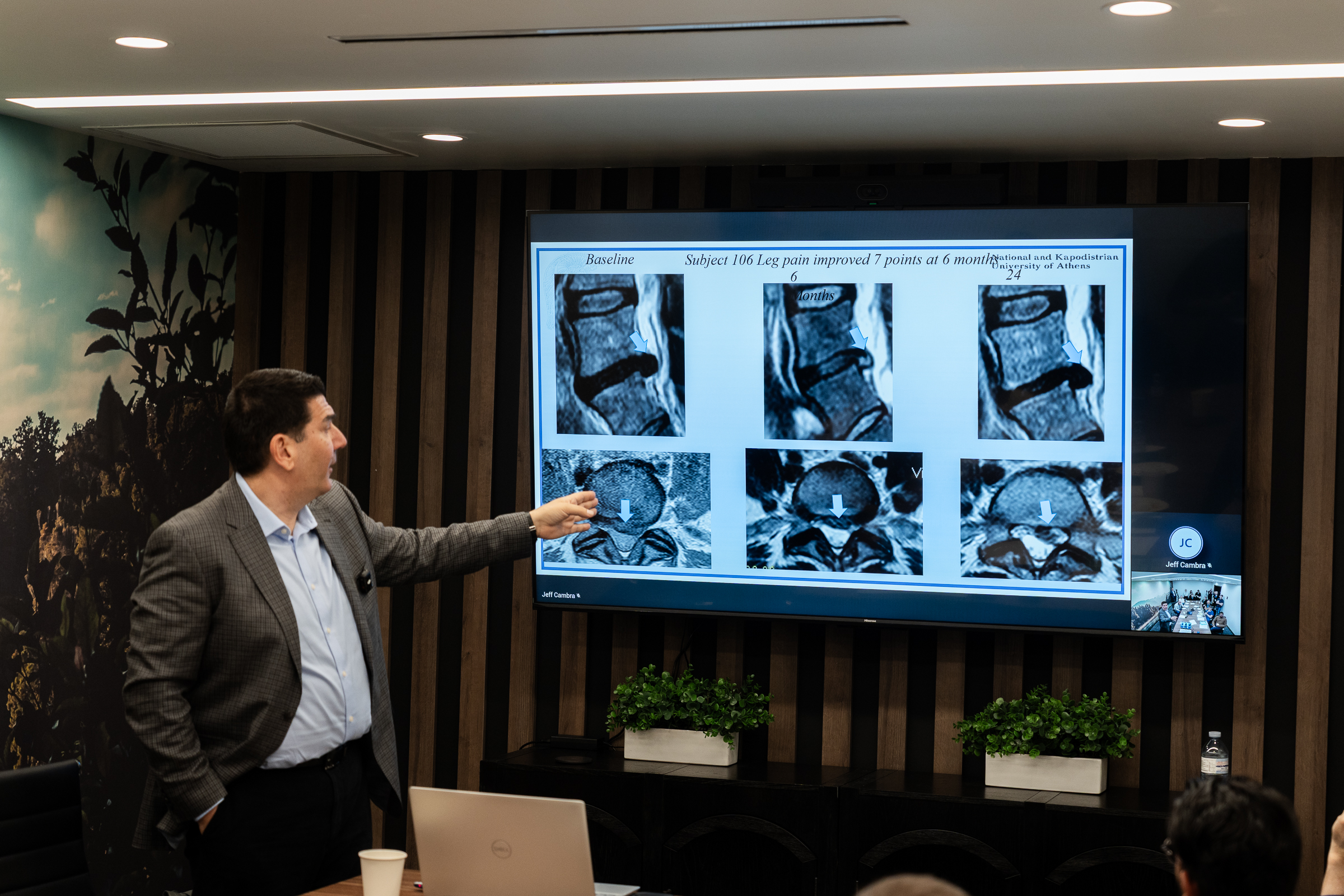
SpinaFX CEO Jeff Cambra was present to support the team and engage with attendees throughout the day. The session also featured an in-depth procedural introduction by John Soloninka, SpinaFX’s Chief Technology Officer. John’s presentation helped frame the clinical and technical aspects of the therapy, providing a strong foundation for the cadaver lab.
Joining the team on-site was Jody Mendez, SpinaFX’s Head of Clinical Affairs, whose presence helped ensure the session ran seamlessly and remained focused on scientific rigor, physician education, and collaborative learning.
Regulatory Compliance
SpinaFX’s therapy is not currently approved in Canada, the United States, or any other country. All procedures and materials demonstrated during the event were presented strictly for educational and training purposes, with reference to Special Access Program guidelines. These activities are designed to support physician learning, foster clinical dialogue, and responsibly introduce new approaches to complex spinal conditions
About SpinaFX
SpinaFX is a Canadian company focused on the development of image-guided, minimally invasive treatments for contained lumbar disc herniations. Through research partnerships, physician education, and thoughtful innovation, SpinaFX seeks to support clinicians in delivering meaningful care for patients affected by spinal conditions.